Chemistry Concept Gk On Full Atmioc Structure, isotope Etc
Atomic Structure Conceptual Chemistry In Hindi
 |
| Atomic Structure |
केमिस्ट्री की महत्वपूर्ण अध्याय परमाणु सरंचना
Dosto Aaj Key Topic Bohot He Important Bisoy Pey Bana Raha Hua Ismey Mey Aapko Atomic Structure , Isotope, Isobar, Isotone Sath He Mukhya Quantum Number Aadi Key Barey Mey Bata Yunga Aur Next Day Mey Isika Part 2 Layunga Jismey Details Mey Aur Bohot He Asani Sey Electron Configuration, Afbau Principal Bistar Sey Aur Sath He Balance Eclectron Aur Valency Tatha Core Electron Key Barey Mey Samjhayunga ,,Hope Ki Issey Aapko Subidha Miley Aur Aap Aaney Waley Exam O Mey Afbal Number Lakey Pass Karey,, Dhanyabad,,
Atomic Structure Conceptual Chemistry In Hindi
Chapter Ko Parney Key Liye Sabsey Pahley Hamey Janana Parega Atom Kya Hey Easy Way Mey Bata Ta Hu aapko Atom Means Tatwa Ka Bo Sabsey Chota Ekai Jo Satantra Abastha Mey Na Rahney Ka Babujud Bhi Chemical reaction O Mey Bhag Leta Hey
Aab Hum Hum Log Jan Giye Ki Atom Hey Kya ,,Aab Aapko Bata Dey Ki Pahley Aaisa Mana Jata Tha Ki Atom Ko Kisi Bhi Condition Mey Tora Nehi Jaa Sakta Par Aaj New Science Sey Yeh Pata Chala hey Ki Atom Ko Tora Ja Sakta Hey Aur Is Ko Bhag Mey Toda Ja Sakta Hey Jo Hey
1. Electron 2.Proton 3.Neutron
Matlab Kya Hua Ki Atom To Ek Bohot Chota Ekai Hey Yeh To Humko Pata Chala Par Tab To Yeh Bhi Sayed Kuch Chotey Particle Sey Jarur Bana Hoga Iska Ans Hey Ha Atom Bhi Kuch Chote Particle Sey Bana Hey Quark Kahtey Hey ,Aur Aapko Bata Dey Ek Tatwa Mey Upasthit Sabhi Quark Aur Quark Sey Banney Waley Atom Ki Gun Dharma , Rasayanik Prabitti Hamesha Ek Hote Hey Jo Us Tatwa Ki Hota Hey
Aur Parmanu Ka Chota Part In Quark Ki Upasthiti Ki Barey Mey Sabsey Pratham Baataye They USA Mul Key Scientist Murgelman ney.
Dosto Sabsey Upar Hum Log Parey They Ki Pahley Scientist Soch Tey They Ki Atom Ko Tora Nehi Ja Sakta Par Aab yeh Proof Hua Hey Ki isko Tora Ja Sakta Hey ,,To IS Sandarv mey Aapko batan Chahunga Ki Atomic Theory Ko Sahi Samajh Ney Key Liye Aaj Tak Bohot Sarey Scientist O Ney Aapna Aapna Atomic Theory Pess Kiye Hey Aur Jissey Atom key Sabhi Gun Dharma Ka Alag Alag Aayam Ka Barey mey Hamey Pata Chala Aaiye Atomic Theory Key Barey Mey Kuch Pramukh Scientist Kya Kahtey Hey Bo Jantey Hey
Dalton's Atomic Theory- Parmanu Ka Barey Mey Sabsey Pahley Jin Scientist Ney Aapna Aabdharna Diye They Bo They Dalton Aur Isi Liye Inhey Atomic Theory Ka Janak Mana Jata Hey,, Inka Hisab Sey Atom Ko Na Nirman Kiya Ja Sakta Hey Aur Nahi Issey Binas Kiya Sakta Hey Aur Nato Isko Bibhajit Kiya Ja Sakta Hey ,,Par Dalton Ka Yeh Theory Aaj Galat Siddha Ho Chuka Hey.
Thomson's Atomic Theory- Thomson's Ki Anusar Parmani (Atom) Ek Dhanabesit Gola Hey Jis Key Charo Aur Negative Charge Ki Electron ki Kan Parikrama Karta Hey Jissey Pura Parmanu Abesh Hin Ya Udasin Ho Jata Hey
Rutherfords Atomic Theory - Rutherfords Ki Anusar Parmanu mey Ek Navik Hota Hey Jo Positive Charge Hotey Hey Aur Negative Charge Us Positive Charge Ki Navik Key Charo Aur Parikrama Karta hey Jiskey Bajasa Sey Unka Yeh Manna Tha Ki Navik Ka Trijja , Parmanu Ki Trijja Sey Jarur Chota Hoga Aur Yeh Sahi Bhi Hey.
Jaisey Aaj Navik Ka Trijja Ka Man Hey 10-15 Meter Aur Parmanu Ki Trijja Ka Maan 10-10 Meter Mana Jata Hey.
Note- Dhyan Dijiye Man Minus Mey Diya Giya Hey Matlab Minus Ka Baad Jitni Bara Sankhya Maan Utna He Chota So Confuse Mat Hoiye.
Dosto Upar Mey Hum Log Aab Ek Neya Terms Ko Suney Jo Hey Navik So Chaliye Aab Navik Key Barey Mey Details Mey Samajh Tey Hey
Dekhiye Dosto Parmanu Ko Maniye Ek Bara Cheez Jiska Bich Ki Bhag Hey Navik Jisko Nucleus Bhi Kaha Jata Hey Aur Is Chotey Navik Mey Parmanu Ka Kul Drabya Man Rahta Hey Aur Sath He Yeh Positive Charge Sey Bana Hua Hota Hey Aur Electron Iskey Charotaraf Ghumtey Rahtey Hey Jissey Ek Akarshan Bal Milta Hey Aur Negative, Positive Ki Aapsi Akarshan Sey Pura Parmanu Udasin Ho Jata Hey ,,Yeh Hey Navik Ka Overview Aab Aapko Navik Ka Kuch Bisheshta Bata Ta Hu
1. Navik Mey Positive Charge Ki Proton Key Sath Sath Chargeless Neutron Bhi Hotey Hey
2. Navik Mey Parmanu Ka Pura Drabya Man Rahta hey
3. Neutron Or Proton Ki Mel Ko Nucleons Kahta Hey
4. Sabhi Nucleons Ki Bich Ek Akarshan Baal Kaam Karta Hey Jissey Navik Ko Sthirta Milta Hey.
Aab Tak Humney Jana Tatwa Sabsey Bara Uskey Andar Parmanu Hey Aur Parmanu Ka Andar Electron , Proton Aur Neutron Hey,, Jo Ek Bebasthit Dhang Sey Rahta Hey Jaha Do Ghar Hey Ek To Hey Navik Jaha Proton Aur Neutron Hotey Hey Aur Itna Jagah Chorkey Parmanu Ka Baki Bhag Mey Rahta Hey Electron Aur Sath He Humney Yeh Bhi Jana Ki Jo Navik Hey Usko Nucleons Bhi Kahtey Hey Kiuki Usmey Proton+Neutron Hota Hey
Lekin Agar Navik Mey Sthit Sirf Proton O Ki Sankhya Ka he Ginti Ho To Usey Kya Kahey ?
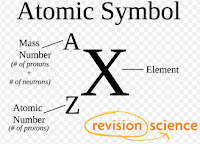
Atomic Number- Koi Tatwa Ki Parmanu Key Andar Navik Mey Sthit Only Proton O Ki Sankhya Ko Us Tatwa Ki Atomic Number Mana Jata Hey Aur Aapko Bata Dey Ek Tatwa Mey Sthit Lakho ,,Arbo Parmanu Key Sabhi Key Atomic Number Hamesha Ek Hota Hey Jiski Bajah Sey Sabhi Alag Alag Tatwa Ki Aapna Alag Alag Gun Dharma Hota Hey Jaisey Koi Tatwa Ko Hum Dhatu Boltey Hey Kisi Tatwa Ko Hum Adhatu Boltey Hey ,,Kisi Tatwa Sey Achcha Khuboo To Kisi Tatwa Sey Hum Kharap Badboo Ki Aas Rakhtey Hey,, Yehi Gun Dharam Ka Pata Hamey Atomic Number Sey He Pata Chalta Hey .
Aur Tatwa Ki Gun Dharma Ki Itna Sathik Jankari Deney Ki Bajah Sey Modern Periodic Table Mey Atomic Number Ki Base Pey He Tatwa Ka Bargikaran Kiya Giya Hey. Jabki Mendeliff Ka Periodic Table Mass Number Pey Banaya Hua Tha Isiliye Us Periodic Table Ko Baad mey Badalna Para Tha Aur Aaj Ka Sathik Modern Periodic Table Banana Para Tha.
Mass Number- Kisi Tatwa Key Parmanu Key Andar Mey Sthit Navik Mey Upasthit Sabhi Proton+Neutron Ki Jor Ko He Us Tatwa Ka Mass Number Ya Drabyaman Sankhya Mana Jata hey Aur Aapko Bata Dey Jo Ki Koi Bhi Tatwa Ka Gun Dharma Uska Mass Number Pey Nirvar Nehi Karta Isiliye Ek Tatwa Mey Sthit Lakho, Aarbo Parmanu Ka Mass Number Ek Bhi Ho Sakta Aur Alag Alag Bhi Ho Saktey Hey. Aur Yehi Sey Suru Hota Hey Isotop, Isobar, Isotone Key Barey Mey
Isotop, Isober , Isotone
Isotop- Jiska Atomic Number Same Hota Hey Par Mass Number Alag Alag Hota Hey
Inkey Rasayanik Gun Saman Aur Voutik Gun Alag Hota Hey
Isober- Jiska Mass Number Same But Atomic Number Alag Alag Hota Hey
Inkey Rasayanik Gun Alag Hotey Hey
Isotone- Jiska Mass Number Aur Atomic Number Dono He Alag Ho Par Nutron Number Same Hota Hey
Man Lijiye Aap 'A' Naam Ka Ek Tatwa Liye Usko Tor Tey Giye Tor Tey Giye Aur Lakho Arbo Tukro Mey Bant Diye Aur Isi Tarah Hazaro Chotey Chotey 'a" Naam Key Parmanu Aapko Mil Giya ,
Is Key Baad Aap Ney Ek Chota Sa 'a' Parmanu Uthaye Aur Usko Torey To Aapney Dekha Usmey 5 Proton Aur 2 Nutron Hey Matlab Aapko Pata Chala Ki Jo Ki 5 Proton Hey Matlab Iska Atomic Number Hey 5 Aur Sath He Jo 2 Nutron Bhi Hey Matlab Mass Number Hua (Proton No.+Nutron No) Matlab (5+2)=7 Yani Mass Number Mila 7 But Aapney Jab Ek Dusra Chota 'a' Parmanu Uthaye To Us Mey Paya Proton To 5 He Hey Par Usmey Nrutron Ki Sankhya 3 Hey Matlab Us Parmanu Ka Atomic Number To 5 Hua Par Mass Number 8 Ho Giya Aur Sabhi Lakho , Arbo Parmanu Mey Hey Aap Ko Sirf In Do He Tarika Parmanu Miley To Isi Sey Aap Samajh Saktey Hey Ki Tatwa "A" Ki Do Isotope Hey Jismey Dono Ka Atomic Number To Same Hey Jo Ki 5 Hey Par Unka Mass Number Alag Alag Hey Jaisey Ek Ka 7 Hey Aur Dusrey Ka 8 Hey , But Agar Koi Dusra Tatwa Aap Tortey Hey Aur Dekhtey Hey Unmey Sthir Sabhi Parmanu O Ka Atomic Number Aur Mass Number Same Same Hey Aur Kisi Ka Bhi Alag Koi Mass Number Nehi To Us Condition Mey Aap Yeh Samajh Saktey Hey Ki Us Tatwa Ki Koi Isotope Nehi Hey.
Isitarah Aap Isober Or Isotone Ko Bhi Imagine Kar Saktey Hey
Aabtak Hum Ney Tatwa Uskey Andar Parmanu Us Key Andar Navik (Protone+Nutron) Aur Navik Ko Parikrama Kartey Huye Electron Key Barey Mey Janey Aab Hum Janegey Ki Navik Key Charo Taraf Jo Electron Parikrama Kartey Rahta Hey Bo Kaisey Ek Niyam Tahat Bebosthit Hota Hey.
Orbit (कक्ष )- Pichey Hum Log Yeh Janey They Ki Ek Parmanu Ka Andar Ek Navik Hota Hey Jis Key Charo Taraf Electron Parikrama Karta Hey But Kya Bo Aisey he Idhar Udhar Bhatakta Rahta Hey Ans Nehi Kiu Ki Electron Ki Kan Hamesha Ek Orbit Bana Key He Parikrama Karta Hey Jaisey Kuch Electron Navik Ka Samney Ek Chota Orbit Mey Ghumta Hey Us Sey Dur Kuch Electron Pahley Wala Sey Thora Bara Orbit Bana Key Ghumta Hey Ussey Baad Bala Ussey Bhi Bara Orbit Bana Key Ghumta Hey ,,Yeh Orbit Ek Khali Jameen Ki Tarah Hota Hey Jaha Electron Aapna Aapna Ek Ghar Bana Key Rahta Hey Isiliye Electron Jitna Jada Hoga Unko Rahney Key Liye Jagah Bhi Utna Barey Chaiye Hoga Aur Aur Utna He Jada Makan Ka Bhi Jarurat Parega
Scince Ki Bhasa Mey Isi Khuli Jammen Ko Orbit Kaha Jata Hey Jisko K,L,M,N etc Mey Bata Jata Hey Aur Jo Makan Key Barey Mey Kaha Usko 'Sub-Orbit' Kaha Jata Hey Jisko s,p,d,f Mey Bata Jata Hey
Principal Quantum Number -Par Ismey Kuch Sart Hey Jaisey Aap Simply Alag Alag Orbit Mey Rahney Wala Adhiktam Electron Ki Sankhya O Ko Nikal Ney Key Liye Ek Formula Use Kar Saktey Ho Jo Hey - 2n2 Jaha 'n'= Orbit ki Number Hey Jaisey 1st Orbit Or 2 Nd Orbit Eaisey.
Aur Aap Upar Ka Formula Ko Use Kar Key Kuch Is Tarah Sey Orbit O Mey Upasthir Adhiktam Electron O Ki Sankhya Ko Nikal Saktey Ho Jaisey Man Lijiye Pahla Orbit Ka Electron Ka Adhiktam Upastiti Nikal Na Ho Tab n =1 Hoga Aur Formula Mey Apply Karengey To Hoga
2n2 { Jaha n=1}
= 2 x 12
=2
2n2 {Jaha n =2 }
= 2 x 22
=2 x 4
8
Isi Tarah Aap Atomic Number Key Wise Orbit Or Alag Alag Orbit O Mey Upasthit Electron Ki Adhiktam Sankhya Ko Nikal Saktey Ho
Lekin Ek Cheez Ka Hamesha Dhyan Rakhna Ki Kabhi Bhi Ek Parmanu Ka Last Orbit Mey 8 Sey Adhik Electron Nehi Ho Sakta .
Jai Sey 2n2 Formula Sey Aapko Pata Chaleyga Ki Is Hisab Sey
k Orbit Mey -2
L mey - 8
M mey - 18
N mey -32
O mey - 50 Electron Ho Saktey Hey
Par Man Lijiye Aap Ney Koi Aaisa Tatwa Liye Jiska Atomic Number Hua 20 Aur Aap Usko Orbit Mey Kuch Is Tarah Devide Kiya
Jaisey Orbit,'K' ko Diye 2 Thik hey
'L' ko Diye 8 Yeh Bhi Thik Hey
Aur Last mey Aap Ney Dekha M Mey To Adhiktam 18 Rah Sakta Hey To Aap Ney Bacha Hua 10 'M' ko Dey Diye But Aap Dhayan Dijiye Aap yehi Pey Galti kar Diye Kiu Ki Yeh Niyam Ka khilaf hey ,,Niyam Ka Hisab Sey Aap Last Orbit Jo Ki Yeha Pey Aap M ko Banaya Usmey Aap Ney 10 Electron Bhar Diye Jab Ki Niyam Key Anusar 8 Sey Jada Electron Aap nehi Rakh Saktey ,, To Aap Is Condition Mey Kya Karengey Aap Ko Niyam Ko Man Tey Huye 'M' mey 8 He Electron Rakhna Parega Aur Baki 2 Key Liye Ek Neya Orbit 'N' Lena Parega Jismey Aapko Bacha Hua 2 ko Bharna Hoga Tab Yeh Yeh Bebastha Sahi Mana Jayega. Hope Ki Aapko Mey Samjha Paya Hu.
But Aapko Batana Chahunga Electron Ko Bharney Ka Yeh Ek Dum Sathik Tarika Nehi Hey Kiu Ki Is Tarika Ko Use Kar Key Aap Chotey Atomic Number Waley Tatwa Ko To Bhar Saktey Ho Par Jab Barey Atomic No Waley Tatwa Aayengey Tab Yeh Tarika Sey Aap Ko Problem Ho Sakta Hey .
Isi Liye Aapko Pahaley Jisko Mankan Kaha Tha Means Sub-orbit Key Barey Mey Janana Hoga ,, Sub-Orbit Ek Tarah Ka Makan Hey Jo Orbit Naam Key Jameen Mey Rahta Hey Aur In Sub-Orbit O Mey He Electron O Ko Bhara Jata Hey Lekin Ek Bebosthit Dhang Sey
Jaisey Orbit Ki tarah he Iska bhi Alag Alag naam Hota Hey Jaisey s,p,d,f, Uskey Baad Aata hey 2s , 2p, 2d, 2f, Is Tarah Sey Isko Pradarsit Kiya Jata Hey
Jaha Alag Alag Sub-Orbi Mey Rahney Wala Adhiktam Electron Ki Sankhya Nimna Rup Hota Hey
|
Sub Orbit
Name
|
Ashiktam Electron
Sankhya
|
| s |
2
|
|
p
|
6
|
|
d
|
10
|
|
f
|
14
|
Hoond Ki Theory -Aur Electron Sub-Orbit Naam Key Andar Alag Alag Room Mey Rahta Hey Aur Hamesha Yugmak Mey Rahta Hey Aur Isko Hoond Ki Theory Kaha Jata Hey Aur Inka Sankhya Nimna Rup Hota Hey
|
Sub Orbit
Name
|
Room
Sankhya
|
| s |
1
|
| p |
3
|
| d |
5
|
|
f
|
7
|
Aafbau Principle - Electron O Ko Ek Sahi Sey Bebasthit Kar Ney Ka Sathik Tarika Hamey Afbau Principle Sey Milta Hey ,, Unhoney Apna Sidhanta Mey Ek Alag Alag Orbit O Ka Urja Pey Dhyan Diya Aur Yeh Bataya Ki Electron Hamesha Kam Engergy Wala Orbit Ko Bharta Uskey Baad Jada Urja Wala Orbit Ko Bharta Hey Aur Isi Tarah Sey Ek Ka Bad Ek Sub-Orbit O Ko Bhartey Jata Hey , Jissey Electron Bhi Sahi Sey Bebasthit Ho Jata Hey Aur Orbit O Ka Yugmak Bhi Pura Ho Jata Hey
To Be Continue......
Note- Dosto Agar Aaj Ka Post Achcha Laga Ho To Pls Isey Sharey Kijiye Aur Agar Apra Hamara Har Ek Neya Post Ka Notification Pana Chahtey Ho To Pls Follow Button Pey Click Kar Key Mujhey Follow Karey ya Phir Nichey E-Mail Box Mey Aapna E-Mail Id Save Kijiye Aap Nischint Rahey Yeh Google Ka Survice Hey Secure And Totally Free He

EmoticonEmoticon