Chemistry Gk Concept on Electronic Configuration , Valence & Valency In Hindi
Important Chemistry Concept In Hindi On Atomic Structure , Configuration
केमिस्ट्री ज्ञान ऑन इलेक्ट्रॉनिक बिन्यास , संयोजी & संयोजकता
Dosto Aaj Ka Chapter Bohot Important Hey Is Post Mey Aapko Details Mey Electronic Connfiguration Sath He Valence Cell, Valency Ki Barey Mey Bhi Sampurna Aur Easy Way Mey Jankari Mileygi Hope Ki Issey Aapko Is Chapter Ko Achchi Tarah Sey Samajh Aayegi. Dhanyabad,,,
Dosto Aab Tak Hum Ney Paramanu Kya Hey Aur Paramanu Tatwa Ki Bibhinna Dharna Ko Samjhey They Aur Sath He Primary Quantum Number And Afbaw Theory Key Barey Mey Thora Baat Chit Kiye They But Aaj Hum Details Mey Is Key Barey Mey Baat Karengey Mey Sure Hu Isko Parney Key Baad Aapko Electron Configuration Karna Bohot He Asan Ho Jayega
Dekhiye Agar Electron Configuration Karna Ho To Kuch Theory Ko Hamey Jarur Samajh Na Hey Nichey Mey Uskey Barey Mey Discuss Kar Raha Hu
Sabsey Pahley Aapko Hamesha Ek Baat Ka Kheyal Rakhna Parega Ki Jo Electronic Configuratin Aap Bana Rahey Ho Usmey Kabhi Bhi Jab Aap Last Orbit Mey Electron Key Bharengey To Ek Baat Ka Kheyal Rakhiye Ki Jaisey Usmey Kisi Bhi Condition Mey 8 Sey Adhik Electron Last Orbit Mey Naa Aa Jaye
2. Electron Configuaration Hamesha Aufbau Principle Key Hisab Sey He Kariyega Issey Kabhi Bhi Aapka Electronic Configuration Galat Nehi Hoga
3 Hamesha Yeh Baat Ka Kheyal Rakhiye Ga Ki Electron Sabsey Pahley Kamjor Orbit O Ko Bharta Hey Baad Mey Strong Orbit O ko Aur Strong Karta Hey
4. Jabtak Sub Orbit O Mey Kam Sey Kam Ek Elctron Na Aa Jaye Tab Tak Ussey Pahley Wala Sub-Orbit Aapna Yugmak Pura Nehi Kar Sakta (Hoond Prnciple)
Aab Mey Ek Easy Example Key Madhyam Sey Isko Samjhata Hu
Example- Ca20 (Calcium)
Electronic Configaration by Primary Quantum Number Or Bohr-Bury Principle
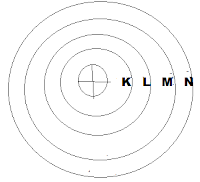 |
| Bohr-Bury Concept With Orbit |
- Is Bidhi Mey Sabsey Pahley Aapko Dekhna Hey Atomic Number Kitna Hey Jo Is Question Mey Hoga Atomic No- 20 hey
So Aab Aapko formula --> 2n2 Ko Use Karkey Dekhna Hoga Kitni Orbit Nikaley Jissey 20 Electron Baat Jaye
So Aap Pahla Qrbit Key Liye n=1 Mankey Nikalengey 2 x 12 So Hoga = 2
Isi tarah n=2 Mankey Nikalengey 2 x 22 So Hoga = 8
Isi tarah n= 3 Mankey Nikalengey 2 x 32 So Hoga = 18
To Aab Aap Sayed Soch Rahey Hongey Yeh To Kul ( 2+8+18) = 28 Ho Ja Raha
But Aap Key Pass To Only 20 Electron He Hey
Yehi Peyn Kaam Hey To Dekhiye Aaisa Condition Mey Aapko Kya Karna Hey
Upar Mey Jo Man Nikla Tha Bo Configuration Kuch Aaisa Tha ---- 2,8, 18
Lekin Dhyan Dijiye Is Mey Last Orbit Mey Only 8 Electron Sey Jada Electron 18 Hey
So Sabsey Pahley To Isi Sey Aapko Samajh Jana Hey Ki Yeh To Galat Hey ,,, Aur Aab Aapko Karna Kya Hey
To Dekhiye Sabsey Pahley To Aapko 8 Electron Chorna Hey Kiu Ki Question Ki Question Mey To Only 20 Electron Ka He Baat Kiya Giya Hey So 28 Mey Sey 8 Nikal Dey To Raha 20
Aab 2n2 Formula
Sey Pahley Itna Orbit Nikaley Jissey 20 Electron Fit Ho Jaye
To Aab Payengey Pahla Mey 2, Dusra Mey 8 Aur Last Mey 10 Yani 3 Orbit Mey Pura 20 Electron Adjust ,,,
Yani Calcium Ka Electron Configuration Aap Nikaley 2, 8, 10
But Dekhiye Aap Phir Sey Galti Kar Rahey Hey ,, Aapko Hamesha Yeh Yaad Rakhna Hey Ki Last Orbit Mey Kabhi Bhi 8 Sey Jada Electron Nehi Rah Sakta But Aap Ney Last Orbit Ko 10 Electron Dey Diye
So Aab Baat Hey To Aap Karengey Kya ,, To Is Condition Mey Aapko Jo Karna Hey Bo Yeh Hey Ki Aapko Ek Aur Neya Orbit Banana Parega Jo Ki 4th Orbit Hoga
2n2 Formula Key Hisab Sey Jiska Electron Capacity Hoga 2 x 42 = 32
But Aap Agar Aapka Banaya Hua Last Orbit Mey Adhiktam 8 Rakhengey To Aapka Pass To Only 2 Electron He Bacheyga ,, 4th Wala Orbit Ko Deney Key Liye
To Aapko 2 Electron Dekey He 4th Orbit Ko Balance Karna Parega Aur Isi Tarah Aap Ek Sahi Electron Configuration Nikal Payengey
So Aab Hamara Calcium Ka Ekdum Sathik Electronic Configuration Kya Nikal Key Aaya
So Nikal Key Aaya Configuration Hoga ------> 2, 8, 8, 2
(Hope Ki Aapko Samajh Aaya Ho)
Electronic Configaration by Azimuthal Quantum Number Or Aufbau Principle
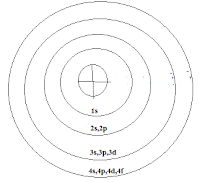 |
| Aufbau Principle With Sub-Orbit Concept |
Is Bidhi Key Madhyam Sey Yeh Kaha Jata Hey Ki Atom Mey Jo Orbt Hota Hey Us Mey Niyam Key Tahat Or Urja Ko Dharan Karney Key Liye Kuch Sub- Orbit Hotey Hey Jismey Electron Bhartey Hey Aur Is Niyam Mey Orbit O Mey Rahney Waley In Sub-Orbit Ko Bharney Ka Order Sey He Electronic Configuration Nikala Jata Hey
Is Niyam Key Tahat Pramukh 4 Sub-Orbit O Ka Baat Kaha Jata Hey Aur Sath He Sabhi Sub-Orbit O Ka Adhiktam Electron Capacity Karney Ka Aakda Bhi Diya Giya Hey Jo Nimna Rup Hey
Sub- Orbit "s" Mey Adhiktam = 2 Electron
Sub- Orbit "p" Mey Adhiktam = 6 Electron
Sub- Orbit "d" Mey Adhiktam = 10 Electron
Sub- Orbit "f" Mey Adhiktam = 14 lectron
{Tricks- Pahley "s' Mey 2 Uskey Baad p, d, f Mey lagatar 4 Ka Barotri}
Aur Sath He Atom Ki Alag Alag Orbit O Mey Alag Urja Hota Hey Isi Liye Sabhi Orbit O Mey Alag Alag Sub-Orbt Rahta Hey
Jaisey
Pahla Orbit Mey Only '1s'
Dusra Orbit Mey -- '2s' & '2p"
Tisra Orbit Mey----- 3's' , '3p' , & '3d'
4th Orbit Mey ------ '4s' , '4p' , '4d' & '4f'
Isitarah Iska Baad Wala Mey 's, p, d, f' to hoga He Sath He Ek Aur 's' Jurega
Matlab Aapko Yeh Samajh Na Hey Ki Har Ek Neya Orbit Ka Trijja Aur Urja Neya Sub-Orbit O Key Jurney Ki Bajah Sey Bohot Jada Hota Hey.
Aab Sayed Aap Soch Rahey Hongey Is Tarah Ho Hum Electronic Configuration Abhi Nikal Lengey Jaisey Calcium Jiska Atomic No-20 Hey Usi Ko Ley Liye Aur Nikal Ney Legey
total -20 Hey
'1s' Ka Adhiktam do to usko Diye - 2
'2s' & '2p' Ka Adhiktam to (2+8) = 8 diye
'3s' , '3p' & '3d' Ya 3rd Orbit Ka Maximm Capacity hey (2+6+10) 18 But Aap 20 Karney Key Liye 10 He Diye Aur Aap Nikal Diye Configuration Hoa ---- 2, 8, 10
But Aapko Batana Chahunga Yeh Galat Hey
Kiu
Kiu Ki Aap Jistarah Matlab Pahley '1s' then '2s' & '2p' then '2s' , '3p' , '3d' & '3f' Key Hisab Sey Electron Ko Bhar Rahey Yeh Order Sahi Nehi
To Kaisey Bharu ?
- Uskey Liye Aap Nichey Is Chirta Ko Dekhey
 |
| Afbaw Princiciple |
Upar Ka Is Chitra Sey Aap Electron Ko Sub-Orbit O Ko Bebosthit Kar Ney Ka Sathik Order Diya Giya Hey Isi Tarah Aap Ko Electron O Ko Bharna Hoga Jis Tarah Arrow Sign Key Madhyam Sey Pahla, Dusra etc Ko Samjhaya Giya Hiye
Aap Chahey To Yeh Order Aap Kahi Draw Bhi Kar Saktey Hey Ya Phir Is Ko Ratta Mar Key Bhi Yaad Rakh Saktey Hey Jaisey Upar Ki Is Picture ki Hisab Sey Electronic Configuration Ka Sahi Order Hoga
Sahi Order - 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d 7p 6f 7d 7f
To Is Hisab Sey Agar Aap Calcium Key Electron Ko Baithayengey To Hoga
1s mey = 2
2s & 2p Mey (2+6) = 8
3s , 3p Mey - ( 2+6) = 8
4s Mey Hoga - 2
Yani Pura 20 Electron Achcha Sey Fit Ho Giya
(** Upar Mey Agar Aap Soch Rahey Ho '3d' Bhi To 3rd Orbit Ka He Bhag To Phir 4th Orbit Wala 's' Ko Kiu 2 Electron Mil Giya ,, '3d' Ko Kiu Nehi Mila To Iska Asan Answer Hoga Ki Sahi Order Key Hisab Sey 4s Tak Electron o Ko Bant Ney Ka Baad Koi Extra Electron Nehi Bacha Tha Isi Liye Hum '3d'wala 3rd Orbit Ka Sub-Orbit Ko Nehi Bharey .
Note- Is Niyam Key Anusar Aapko Hamesha Sahi Order Key Hisab Sey He Electron Varna Hota Hey So Is Baat Ka Yaad Rakhna.
Hoond Ka Niyam -
Critical Ya Bara Atomic Number Waley Tatwa Ka Sahi Electronic Configuration Karney Key Liye Hoond ka Niyam Ko Janna Bohot He Abasyak Hey ..
Is Niyam Key Jariye Yeh Bataya Giya Ki Jab Tak Koi Ek Sub-Orbit uska Up-Kakshya O Ko Minimum Ek Ek Electron Nehi Dey Pata Tab Tak Us Sey Pahley Wala Sub-Orbit Aapna Yugmak Pura Nehi Kar Sakta
Aur 's' , 'p' , 'd' & 'f' Ka Upkakshya O Ki Sankhya Nimna Rup Hota Hey
's' Sub Orbit Ka Upkakshya Ki Sankhya = 1 Jora
'p' Sub Orbit Ka Upkakshya Ki Sankhya = 3 Jora
'sd Sub Orbit Ka Upkakshya Ki Sankhya =5 Jora
'f' Sub Orbit Ka Upkakshya Ki Sankhya = 7 Jora
{Tricks - 's' Ko Yaad Rakhiye Uskey Bad 'p' , 'd' & 'f' Key Liye Ek, Ek Jor Lijiye}
To Aaiye Isko Ek Example Ki Madhyam Sey Samajh Tey Hey
Iskey Liye Hum Letey Hey Isko
Example : Cr24
(Chromium)
Matlab Atomic No - 24
Aap Sabsey Sahi Afbaw Principle Sey Nikaley
Aur Is Order Sey Baithaye
1s, 2s , 2p ,3s ,3p , ,4s 3d
Mean - 2 2 6 2 6 2 4 (Remaining)
Matlab Aap Ney Configuration Nikaley
1s, 2s + 2p , 3s +3p+3d, 4s
= 2, 8, 12, 2
But Yeh Galat Hey Kiu Agar Aap Dhyan Dengey To Aapko Pata Chaleyga Jab Aapney Sahi Order Key Hisab Sey Electron O Ko Sub-Orbit Mey Varey They To Aap Ney '3d' Sub-Orbit Ko Bacha Hua 4 Electron He Diye They But Aap Pahley Yeh Par Chukey Hey Ki Sabhi Sub-Orbit O Ka Alag Alag Up-Kakshya Bhi Hota Hey Jsimey Sabhi Kakshya Yugmak Abastha Mey Rahna Chahta Hey But Agar Electron O Ki Kami Ho To Us Condition Mey Sabhi Up-Kakshya O Ko Kamsey 1 Electron Jarur Dena Parta Hey Aur Agar Us Key Upkakshya Ko Bharney Layek Electron Na Ho To ,,, Us Sub-Orbit Sey Pahley Wala Sub-Orbit Ka Yugmak Torkey Uska Sabhi Up-Kakshya O Mey Minimum Ek, Ek Electron Varna He Parta Hey.
Aab Aap Upar Ka Configuration Ko Dekhiye Jsmey Aapney
Last Wala Sub- Orbit Ko Only 4 Electron He Dey Paye They Jabki Hum Pahley He Yeh Jan Chukey Hey Ki Sub-Orbit 'd' Key Liya Upkakshya Ki Sankhya= 5 Jora Hey Ya Muskil Sabhi Upkakshya Mey Agar Ek, Ek Bhi Electron Dey , Phir Bhi Minimum 5 Electron Ka Jarurat Hey But Aap Key Pass To Only 4 Electron He Hey To Ek Aur Electron Kaha Sey Layogey ,,
Is Key Liye Aapko '3d' Sey Pahley Wala Yani '4s' Jiska Bartaman Mey 2 Electron Key Jariye Yugmak Pura Ho Chuka Hey Uska Yugmak Torna Hoga Aur '4s' Sey He Ek Electron Lena Hoga Yani Aab '3d' Key Pass Kul 5 Electron Ho Giye Aur ,,, '4s' Sub-Orbit Sey Ek Electron Ley Leney Key Bajah Sey Uskey Pass Aab Only 1 Electron He Bacheyga
So Aab Agar Phir Sey Ekbar Electron Configuration Nikalna Chahey To Usmey Sahi Order Mey Sabhi Sub-Orbit O Mey Electron Ki Sankhya Kuch Is Tarah Hoga
Sahi Order - 1s 2s 2p 3s 3p 4s 3d
Means- 2 2 6 2 6 1 5 ('4s' sey 1 Electron Milney Key Baad)
Matlab New Configuration Hoga
1s, (2s+2p), (3s+3p+3d), 4s
= 2 (2+6) (2+6+5), 1
= 2, 8, 13, 1
Valence Electron -- Balance Electron, Koi Electronic Configuration Ki Last Mey Jo Sankhya Hota Hey Usey he Balance Valance Electron Kaha Jata Hey Jaisey
Upar Mey Hum Log Jo Chromium Ka Electronic Configuration Nikaley Usika Last Sankhya
Jaisey Hamara Configuration Nikla Tha == 2, 8, 13, 1
Matlab Yeha Pey last Ka Number Tha = 1
So Yeha Balance Cell No Hua = 1
Valency- Dosto Valance Electron Ki Jo Sankhya Hota Hey Usiko 8 Sey Minus Kar Ney Sey Hamey Jo Parinam Milta Hey Bo He Us Tatwa Ki Valancy Hota Hey
But Ismey Dhyan Rakheny Wala Yeh Hey Ki Agar Aapka Configuratin Ka Last Number Matlab Valance Electron Ki Sankhya 1, 2 , 3 Ho To Us Condition Mey Valency Bhi +1 , +2 & +3 Hoga
But Agar Valence Electron Sankhya 4, 5 , 6 , 7 , 8 Hoto Hey To, 8 Sey Aapko Un Sankhya O Minus Karna Parega Aur Us Sthiti, Mey Parinam Ka Sign Bhi Aapko Minus Mey He Lena Parega
Jaisey Agar Koi Tatwa Ka Valency Electron Ki Sankhya Hua - 5
To Us Tatwa Ka Valancy Hoga (8-5) = --3 (Minus Three)
Core Electron- Kisi Tatwa Ki Electronic Configiration Mey Last Sankhya Ko Chorkey Baki Sabhi Ko Core Electron Sankhya Kahtey Hey Jaisey
Agar Kisi Sankhya Ka Electronic Configuration Hua
2,8,8,2
To Uska Valence Electron Sankhya Hoga = 2
Valency Hoga = +2
Core Electron Ki Sankhya Hoga Valance Electron Sankhya Ko Chorkey Baki Sankhya
Jaisey Yeha Core Electron Sankhya Hoga = (2+8+8) = 18
Note- Dosto Aaj Ka Post Aap Sabhi Ko Kaisa Laga Comment Mey Yeh Jarur Batana Agar Achcha Laga Ho To Is Post Ko Share Jarur Karna Sath He Aap Logo Batana Chahunga KI Agar Aap Mera Har Ek New Notification Aapka Mobile Pey Pana Chahtey Ho To Aap Follw Button Pey Click Kar Key Mujhey Follow Karey Ya Phir Nichey E-Mail Box Pey Aapka E-mail Id Submit Karey Aap Nischit Rahey Yeh Dono He Service Google Ka So Yeh Free Bhi Hey Aur Issey Aapki Privacy Ka Koi Luksan Nehi Hoga Yeh Sirf Notification Paney Ka Ek Bebastha ,, Bus Aur Kuch Nehi.

EmoticonEmoticon